Abstract
Background
TEAMM (Tackling EArly Morbidity and Mortality in Myeloma) was a randomised, double-blind, placebo-controlled multi-centre phase III clinical trial assessing the benefits of antibiotic prophylaxis and its effect on healthcare associated infections. Infection in the first 12 weeks is the biggest cause of the high early death rate in myeloma. Levofloxacin is a quinolone antibiotic that is effective against the common bacterial infections in myeloma and taken once daily has proven efficacy as prophylaxis during therapy for other cancers. There has however been concern about the development of antibiotic resistance. We randomised the use of levofloxacin or placebo for 12 weeks to see if levofloxacin reduces febrile episodes and death in patients with newly diagnosed myeloma. Subjects were regularly screened for carriage of resistant organisms to detect if levofloxacin increases their carriage.
Methods
Patients were eligible if >21 years old with newly diagnosed symptomatic myeloma, intention to treat myeloma actively, and were +/- 14 days into a programme of anti-myeloma treatment. Patients were randomised to receive 500 mg levofloxacin or placebo tablets once daily for 12 weeks, dose adjusted for renal function. Patients were permitted to continue routine non-bacterial antimicrobial prophylaxis, including thrice weekly sulfamethoxazole-trimethoprim (SMZ-TMP) for pneumocystis. Faecal and throat samples were taken every 4 weeks to detect carriage of Clostridium difficile, MRSA and faecal ESBL-positive Gram-negative bacteria (ESBLGnB). The primary endpoint was the number of febrile episodes (defined as an oral temperature of ≥38°C treated with anti-infectives) and or death by any cause suffered in the first 12 weeks obtained using Kaplan-Meier curves censored at 12 weeks. Secondary outcomes included death, the number of clinically documented total infections, episodes of severe sepsis and suspected infections. Occurrence of febrile episodes was captured at clinic visits every 4 weeks up to 12 weeks. All patients were included in an intention to treat analyses.
Results
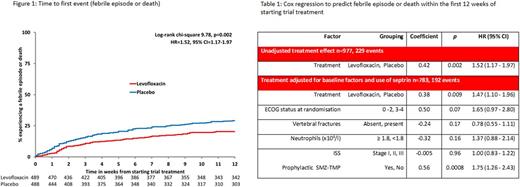
TEAMM recruited 977 patients between August 2012 and April 2016 from 92 centres within the UK. Median age was 67 years, 63% male, 76% had eGFR>50 ml/min, 54% had planned high dose chemotherapy with autologous-stem cell transplantation, 93% were ECOG performance status 0 to 2, 71% presented with bone disease. The primary endpoint showed a significant benefit for the use of levofloxacin with 134 of 488 patients (27%) on placebo reporting events (112 febrile episodes; 15 deaths; 7 febrile episodes and death) versus 95 of 489 patients (19%) on levofloxacin (87 febrile episodes; 4 deaths; 4 febrile episodes and death); hazard ratio (HR) 1.52 (95%CI 1.17-1.97) p=0.002, see figure 1. After 52 weeks there was no survival benefit between arms (p=0.94). Of the 586 total infections, there were 329 infections from 214 patients on the placebo arm and 257 infections from 189 patients on the Levofloxacin arm (chi-square=7.55,P-trend=0.006) with differences emerging after 4 weeks. C. difficile carriage at baseline was uncommon (7 of 785 subjects). Levofloxacin significantly reduced the number of recoded invasive gram-negative infections, but not the number of reported Gram positive infections. SMZ-TMP (315 patients) significantly reduced the number of febrile episodes and deaths and the effect of SMZ-TMP was additive with the effects of levofloxacin. Cox regression adjusting for baseline factors showed levofloxacin treatment (HR=1.47, 95%CI=1.10-1.96, p=0.009) and prophylactic SMZ-TMP (HR=1.75 (95%CI=1.26-2.43, p=0.0008) to be significant predictors for reduction of febrile episodes or death within the first 12 weeks of starting trial treatment, see table 1. There was no significant difference between the 2 arms for carriage or infection with C. difficile, MRSA and ESBLGnB.
Conclusion
Prophylactic use of 12 weeks levofloxacin for patients undergoing treatment for active myeloma significantly reduces febrile episodes and deaths without increasing healthcare associated infections or carriage of key nosocomial pathogens. The value of adding SMZ-TMP to levofloxacin and for periods greater than 12 weeks needs to be explored in future trials.
Funding: Project funded by NIHR HTA programme (08/116/69). Views expressed are those of the authors and not those of the HTA programme, NIHR, NHS or the Department of Health.
Drayson: Abingdon Health: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Bowcock: Celgene: Other: Educational support; Amgen: Honoraria; Takeda: Other: educational support. Yong: Janssen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal